
CARAQA Program

Faced with the evolution of CA/RA/QA functions, the aim is to develop a broad range of technical and human skills in order to evolve towards the organisation’s decision-making centres and play a key role in maintaining the company’s competitiveness and sustainability.
The CAS CARAQA develops the following skills:
- Optimal preparation for MDR 2017/745 and IVDR 2017/746 regulatory changes
- Strategic planning and management of clinical evaluations, investigations under ISO 14155 and IVD performance studies
- Strategic and tactical communication for interaction with Notified Bodies and National Competent Authorities and to deal with crisis situations
- Management and engineering support during new product development projects
- Leadership in the deployment and maintenance of Quality Management Systems under ISO 13485 and US QSR
- Structuring of supply chain, production and marketing structuring
- Technical expertise in key subjects such as risk management, biocompatibility, usability, and software validation, according to current standards
Duration
The CARAQA training average duration is of 200 hours over 6 months
Classes
Classes are hold once or twice per week in loco for the entire day
Exam
An exam covering the whole program is planned at the end
CARAQA Thesis
Participants will write and present an individual thesis on a pre approved subject
Diploma
A diploma will be handed in a formal ceremony as a sign of program completion
Structure
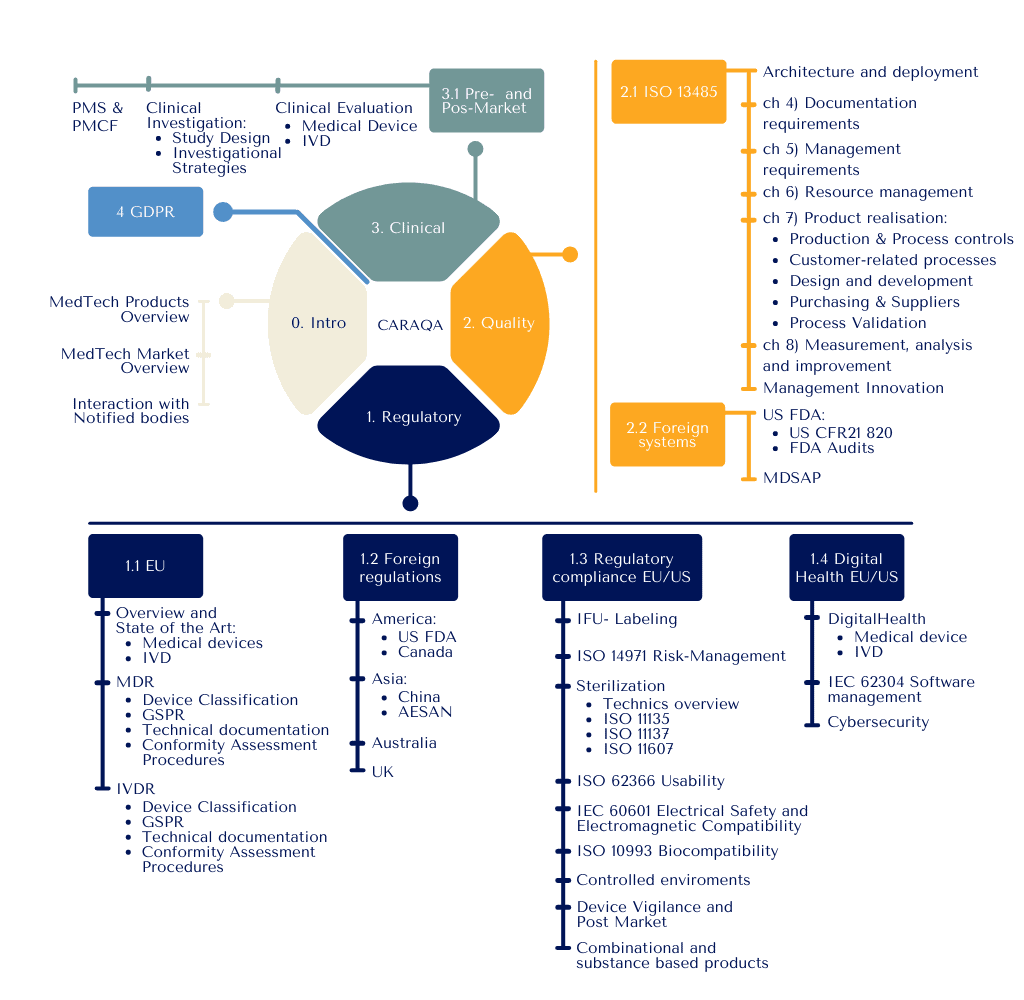
Introduction to the world of devices
-
Understanding the MedTech environment
-
Getting to grips with product life cycle
-
Positioning and interacting with the entities involve
Regulatory Affairs
-
Integrating regulatory requirements during product design and product life cycle
- Managing implementation of directives, standards, recommendations, and software compliance
-
Structuring and implementing regulatory submissions documentation and risk management
-
Managing market events: incidents, reporting, recalls
Quality Assurance
-
Structuring the Quality Management System deployment
-
Organizing documentation and its evolution
-
Supervising process control and controlling suppliers
-
Managing critical quality processes such as audit, improvement, changes
Clinical Affairs
-
Structuring and organizing clinical / performance evaluations
-
Organizing a clinical investigation
-
Performing a literature review
-
Managing post-marketing studies
Target audience
The program is aimed at people in medical device and in-vitro medical device companies (as well as subcontractors) who are facing direct or indirect challenges in a CA/RA/QA environment.
-
Employees in the regulatory, clinical and / or quality assurance department
-
Engineers from electronics, mechanical or software disciplines, in charge of medical device or in-vitro medical device projects
-
Experts in manufacturing and production
-
Physicians, scientists or inventors of medical products
-
Laboratory assistants involved in the development of new analytical methods or process automation
-
Employees involved in clinical studies or quality/regulatory processes in a healthcare organization
Admission criteria
Formal entry qualifications:
- Tertiary Educational Qualification (at least Bachelor’s level) and relevant work experience
- or Diploma HF (from a Swiss “Höhere Fachschule”) and relevant work experience
As the instruction and educational materials are in English, proficiency in English (reading and writing) is a prerequisite.
Specific Admissions if the applicant does not qualify as per formal criteria above:
-
At least 3 years’ work experience corresponding to or related to the relevant Continuing Education Program.