CARAQA
Certified Course of Advanced Studies designed to prepare Regulatory, Clinical, and Quality experts in the MedTech industry.
Our program offers a unique blend of theoretical knowledge and hands-on practice, enabling professionals to navigate complex EU and US regulatory landscapes.
Delivered by state-accredited engineering schools, CARAQA is an on-the-job training that not only meets the competence requirements of the MedTech industry but also helps manufacturers and authorized representatives fulfil the EU Article 15 obligations by identifying a person responsible for regulatory compliance (PRRC).


Certified Training Program

Multiple Locations

Person Responsible for Regulatory Compliance
Supports compliance with the EU Article 15 by identifying a PRRC
The Program
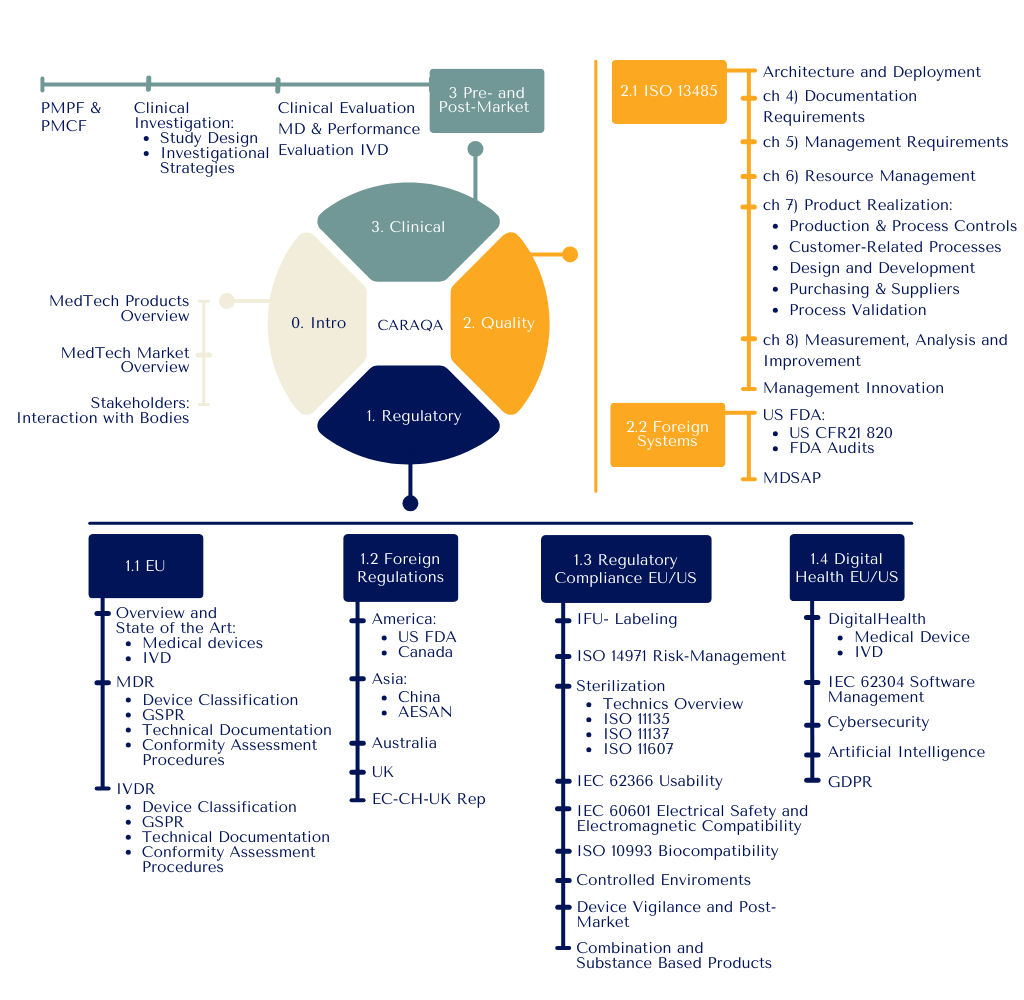
Our goal is to cultivate adaptable leaders who can drive organizational strategy, maintain competitive edge, and ensure sustainable growth in the complex MedTech ecosystem. In response to the rapidly evolving Clinical Affairs, Regulatory Affairs, and Quality Assurance (CA/RA/QA) functions, the CARAQA program is meticulously designed to empower professionals with a comprehensive skill set that transcends technical expertise:
-
Optimal preparation for MDR 2017/745 and IVDR 2017/746 regulatory transitions
-
Strategic planning and management of clinical evaluations, investigations, and performance studies under ISO 14155 and IVD standards
-
Advanced communication strategies for effective interaction with Notified Bodies and National Competent Authorities, including crisis management skills
-
Management and engineering support throughout new product development projects
-
Leadership in the deployment and maintenance of Quality Management Systems aligned with ISO 13485 and US QSR requirements
-
Strategic structuring of supply chain, production and marketing processes
-
Technical expertise in critical domains such as risk management, biocompatibility, usability, and software validation, ensuring compliance with current standards
Duration
Classes
Exam
Thesis
Participants will write and present an individual thesis on a pre-approved subject
Diploma
A diploma will be handed in a formal ceremony as a sign of program completion
Structure
Target Audience
The program is strategically designed for MedTech professionals navigating the complex landscape of Clinical, Regulatory, and Quality Affairs across medical device and in-vitro medical device industries:
-
Regulatory, clinical, and quality assurance department professionals seeking to enhance their strategic capabilities
-
Engineers in electronics, mechanical or software disciplines, in charge of medical device or in-vitro medical device projects
-
Manufacturing and production experts looking to deepen their regulatory and quality understanding
-
Physicians, scientists and inventors developing innovative medical products
-
Laboratory professionals involved in developing new analytical methods or process automation
-
Healthcare organization employees engaged in clinical studies or quality/regulatory processes
Admission Criteria
Formal entry qualifications:
- A tertiary educational qualification (minimum Bachelor’s level) with relevant work experience
- or a Diploma HF (from a Swiss “Höhere Fachschule”) with relevant work experience
Specific admissions if the applicant does not qualify as per formal criteria above:
- At least 3 years’ work experience corresponding to or related to the relevant Continuing Education Program.
Additional prerequisites:
- Proficiency in English (reading and writing), as all instruction and educational materials are delivered in English
- Program coordination by Veranex Switzerland SA
Choose your CARAQA
CARAQA programs are currently available in two of the most dynamic European medtech markets: Switzerland and Belgium. Secure your place in the next session:
Testimonials

“
Regina Orzekowsky-Schroeder
CARAQA Alumna 2021 - PRRC, EUROIMUNN

“This course revolutionized my career by providing essential skills for success in the dynamic field of CA/RA/QA.
It covered vital topics like MDR 2017/745, IVDR 2017/746, RISK management, and product development. The practical on-the-job training and collaboration with Veranex were invaluable. Its recognition by RAPS Switzerland and SWISS MEDTECH enhances its credibility.
I highly recommend this course to professionals in regulatory, clinical, and quality departments.”
Pragash Vijay
CARAQA Alumnus 2021 - Consultant in Medtech for Medical Devices & IVD, PRRC

“After more than 20 years in QA/RA in chemical industry, I have attended the CARAQA programm in 2021/2022. Thanks to this program given by professionals of Medical Device Industry, I have acquired the knowledge needed to join a very dynamic company creating innovative therapies.
The CARAQA programm has been a key enabler of my career transition.”
Nathalene Hippolite
CARAQA Alumna 2022 - Quality Asurance & EHS Manager, Onward
Meet the CARAQA Managing Team
The CARAQA team brings together a powerful consortium of distinguished educators and industry experts, including professors from HEIG-VD in Yverdon-Les-Bains, the School of Life Sciences FHNW in Basel-Muttenz, UCLouvain in Louvain-La-Neuve, complemented by the extensive industry insights of Dr. Elena Lucano and Zuzana Robin from Veranex Switzerland SA.

Prof Didier Maillefer
School of Management and Engineering, HEIG-VD

Prof David Hradetzky
School of Life Sciences FHNW

Prof Renaud Ronsse
UCLouvain

Dr Elena Lucano
Training Manager, Veranex Switzerland SA




